
Divide the number you want to express as a percentage by the total. How do I calculate the percentage? Find out the total or total amount.

How do you find the percentage of a total amount? For example, if you're looking for 50 percent, you'd type 50/100. The required percentage is the numerator and 100 is the denominator. Enter the percentage you want to find as a fraction. How do you calculate a percentage of a whole number?įinding the percentage of an integer is quite easy. Click the Numbers category and then select the Percentage tab. To convert values to percentages: select the desired cells. How do you turn a number into a percentage? There are two common ways to think about what 1 ppm means: a) It is one millionth of a gram per gram of sample solution. The term 1 ppm means that a particular solute is present at a concentration of one part per million of a solution. What does the concentration of 1 ppm mean? To find the PPM in parts per billion, simply multiply the PPM by the conversion factor. How much is 1 ppb as PPM?ġ ppb = ppm Therefore millions are in one part per billion. One ppm corresponds to a total of 1/1000000: 1 ppm = 1/1000000 = 1 × 10 6 One ppm corresponds to: 1 ppm =. Micrograms per liter (μ/L) Unit to measure the concentration of a component in water or wastewater. This measurement is equal to parts per billion (ppb). One thousand micrograms per liter is equal to 1 milligram per liter. Use the following equation to convert units from mg/L to ppm. While ppm is the volume/volume ratio or the mass/mass ratio, mg/L is the mass/volume ratio. The unit of PPM can be converted to mg/L by multiplying by. One part per million equals one milligram per liter. A reading of 6 mg/L corresponds to 6 ppm or 6000 ppb, which corresponds to 6000 g/L.

1 ppm = mg/lįor water, 1 ppm = approximately 1 mg/L (also called mg/L) of contaminants in the water and 1 ppb = 1 μg/L (also called g/L). For example, for chloride, the molar mass of a solution with a concentration of 1 M has g chloride per 1 liter of solution. To convert molarity to ppm, you must first determine the molar mass of the substance. Divide the mass of the first solid in milligrams by the mass of the second solid in kilograms. To calculate the PPM of one solid mixed with another, compare the masses of the two. That is, ppm = (mass of solute ÷ mass of solution) x 1,000,000. The concentration in parts per million or ppm is very similar to the weight percentage, except it multiplies the mass ratio by 1,000,000 instead of 100. How do you calculate the concentration of ppm?
#How to calculate ppm how to
How to convert a number from percent (%) to parts per million (ppm). This number is then multiplied by 106 and expressed in parts per million (ppm). To find parts per million, divide the mass of the solute by the total mass of the solution. What is the formula for parts per million?
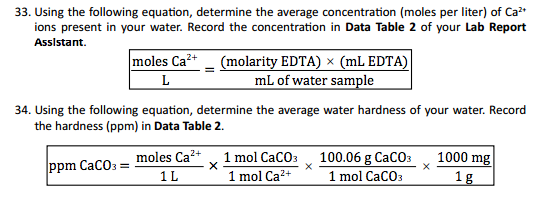
Divide the mass of the solute by the total mass of the solution.
#How to calculate ppm plus
The total mass of the solution is equal to the mass of the solvent plus the mass of the solute. Find the total mass of the solution in g. Measure the mass of the solute you want to mix with your solution. Method 2 of 3: Find the concentration in percent or parts per million. How they can calculate the concentration in ppm? So one part per million is a percentage: 1 ppm = To convert ppm to percent, divide ppm by 10,000: x (%) = x (ppm) / 10,000. How to Convert PPM to Percentage How to Convert Parts Per Million (ppm) to Percentage (%). What does 1 ppm equal? One ppm is equal to 1 milligram of something per liter of water (mg/L) or 1 milligram of something per kilogram of soil (mg/kg).


 0 kommentar(er)
0 kommentar(er)
